Hereditary Hemorrhagic Telangiectasia
Management
Screening for AVMs
Visceral organ AVMs can develop in utero and be present at birth or develop during the first decade of life. Unlike epistaxis and telangiectasias, development of visceral organ AVMs does not appear to be age dependent as the prevalence of AVMs was no different in a recent study of children and adolescents compared to historical data on prevalence in adult populations.29 Current treatment guidelines recommend screening for brain and pulmonary AVMs in HHT patients from the time of diagnosis, even if HHT is diagnosed in childhood.31
Screening for brain AVMs with an MRI with and without contrast is recommended at the time of diagnosis of HHT. If HHT is diagnosed in adulthood and the MRI does not reveal brain AVMs, no further brain screening is recommended. However, if HHT is diagnosed in childhood, repeat screening for brain AVMs should be considered in adulthood even if screening in childhood was negative.
Screening for PAVMs is recommended at the time of diagnosis of HHT. This can be pursued using an echocardiogram with saline contrast (delayed ‘bubble study’) or a CT scan of the chest. A delayed contrast echocardiogram is the preferred initial approach as it is very sensitive for the detection of PAVMs and is not associated with risks related to radiation exposure or use of contrast dye. If the contrast echocardiogram is positive, a chest CT is indicated to determine the location, number and size of PAVMs. The chest CT can be a contrast scan or a high resolution, non-contrast study (in a patient without preexisting PAVMs). If PAVMs are identified, embolization for closure of PAVMs should be considered if they meet size criteria (discussed under treatment of PAVMs). Unlike brain AVMs, screening for PAVMs is recommended every 5 years, as these can develop at any time in an individual’s life.
Screening is not recommended for liver AVMs or for GI telangiectasias unless patients are symptomatic.
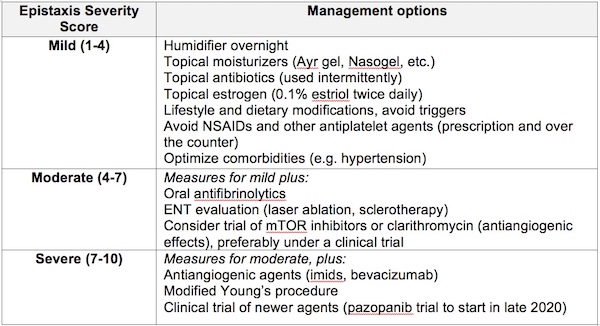
Recurrent epistaxis
Management of recurrent epistaxis is oftentimes challenging and is best approached in a multidisciplinary manner with incorporation of lifestyle modifications, topical measures, and pharmacological and surgical treatments to achieve symptom control. The epistaxis severity score (ESS) is a useful tool to help guide therapy and assess response to treatments.32 The ESS is an online tool that uses a weighted scale to calculate epistaxis severity in the preceding 3 months based on six parameters: frequency, character and duration of nosebleeds, presence of anemia, and need for medical attention, and need for blood transfusions due to epistaxis (https://www2.drexelmed.edu/HHT-ESS). ESS scores of ≤4 are considered mild, scores of >4 to ≤7 as moderate, and scores ˃7 as severe epistaxis.
Treatment of epistaxis can be approached under two broad categories – measures to control acute episodes and ongoing treatments aimed at decreasing frequency and severity of future episodes (prevention/symptom control).
Acute treatment:
The goal of acute management of epistaxis is primarily to achieve hemostasis and stop the bleeding. A number of different approaches may be pursued, and it is our experience that most adults with HHT often have their ‘go to’ methods for this purpose. Approaches include pinching of the nostrils to apply pressure, nasal packing with sterile swabs/gauze, use of commercially available products for nasal packing and tamponade (e.g. Rhino Rocket, BleedArrest), and topical agents that aid in hemostasis (also commercially available, e.g. NexStat). Depending on the severity of the bleed, patients may need to go to an emergency room for further management/packing. Evaluation by an ENT physician and cauterization may at times be necessary to stop an acute nosebleed. While some patients use oxymetazoline nasal sprays to help stop nosebleeds, we do not recommend routine use of this medication given the risk for developing rhinitis medicamentosa (rebound congestion) with repeated chronic use.
Prevention and control:
Approaches aimed at prevention of epistaxis is key in HHT. All patients with recurrent epistaxis should use a humidifier overnight to prevent dryness of the nasal mucosa and prevent/minimize early morning nosebleeds. We also recommend a topical moisturizer to the nostrils 3-4 times daily.33 There are a number of over-the-counter preparations available for this, such as Nasogel and Ayr gel. Gel based preparations are preferred over saline nasal sprays as the former tend to provide more sustained moisture. There are also data that oil-based preparations like rose geranium oil are helpful in decreasing nosebleeds. Topical estrogen (0.1% estriol) compounded in petrolatum applied twice daily (0.25 mL to each nostril) has been shown to be effective as well. Some patients apply antibiotic ointments to the nostrils twice daily. We recommend using this intermittently and alternating antibiotics with other topical moisturizers to prevent antibiotic resistance. It is important to counsel patients to avoid medications and supplements that can potentially worsen epistaxis, such as omega-3, fish oil, garlic, NSAIDs, aspirin, and other agents with platelet inhibitory properties. It is common to see patients who have learned to avoid certain foods and beverages based on individual experience with worsening of epistaxis after consuming them. Lifestyle and job related modifications can be helpful, such as limiting the amount of weight the patient carries, avoiding bending down to pick-up objects, etc.
A number of medications have shown efficacy in the management of bleeding from mucosal telangiectasias in HHT, be it epistaxis or GI bleeding. These include hormonal treatments, antifibrinolytics and antiangiogenic strategies. There is evidence from single center studies and small case series that estrogen-containing oral contraceptives and selective estrogen receptor modulators are effective in decreasing epistaxis in HHT.34 Tamoxifen also seems effective in controlling mucosal bleeding. Hormone modulation approaches confer an increased risk for thrombosis and this will need to be considered, as therapeutic anticoagulation in patients with HHT is complicated given increased risk for hemorrhage.
Antifibrinolytic agents (aminocaproic acid and tranexamic acid) have been used in patients with HHT for a number of years. Recent prospective trials showed a benefit from these agents with significant decrease in severity of epistaxis.35 While the risk for thrombotic complications are frequently raised with the use of these drugs, there are no data to support these concerns. We do not recommend their use in patients with genitourinary bleeding or atrial fibrillation.
Antiangiogenic agents have also shown promise in HHT. The first group of drugs from this class to be evaluated in HHT were the IMiDs (immunomodulatory drugs), with thalidomide showing efficacy in controlling bleeding in HHT patients.36 The use of thalidomide can be limited due to its adverse effect profile. Later generation IMiDs, such as lenalidomide and pomalidomide have a more favorable adverse effect profile and may be better tolerated by HHT patients. More recently, the vascular endothelial growth factor inhibitor Bevacizumab was shown to be effective in controlling both epistaxis and GI bleeding in HHT.37 The durability of response seems limited and continued use of the drug for ‘maintenance’ is needed. The are no data on long-term safety of bevacizumab in HHT patients. There are ongoing efforts to systematically evaluate other antiangiogenics like pazopanib in HHT.38 Anecdotal reports on this agent have been favorable.
Surgical approaches for management of epistaxis include electrical or chemical cauterization, endonasal laser ablation, sclerotherapy, dermatoplasty, embolization of feeding arteries, and the modified Young’s procedure.39-45 We do not recommend electrical or chemical cautery except in emergent situations. The preferred approach is to pursue elective laser ablation of nasal telangiectasias or sclerotherapy when topical and medical approaches are inadequate.
We use a step-wise approach for the management of epistaxis and use the ESS to guide the approaches implemented for prevention and control (Table 1). A collaborative approach with ENT is essential to achieve optimal control of epistaxis.
*adapted from Pahl & Kasthuri, Clinical Hemostasis and Thrombosis, 4e 2018
Treatment of AVMs
Brain AVMs:
There is a paucity of data on the optimal approach to treatment of brain AVMs in patients with HHT. Management is dependent on individual patient characteristics, such as number, size and location of AVMs, comorbidities, etc. The risks and benefits of intervention versus observation should be considered on a case-by-case basis. Embolization, surgical resection, and stereotactic radiation are all available therapeutic approaches to treat brain AVMs in HHT patients.31
Pulmonary AVMs:
The HHT treatment guidelines recommend embolization and closure of PAVMs with a feeding artery diameter of >2.5-3 mm. Embolization and closure of PAVM can be pursued using coils or Amplatzer plugs.31 A follow-up CT scan to evaluate for recanalization of the embolized AVM is recommended 6-12 months post procedure. If no additional PAVMs are present, the patient should revert to PAVM screening every 5 years.
Liver AVMs:
The most feared complication of liver AVMs is high output heart failure. If heart failure is present, catheterization with measurement of cardiac index and pulmonary pressure should be pursued. Liver biopsy is generally avoided because of the high risk of hemorrhage. Potential approaches for the treatment of symptomatic liver AVMs include hepatic artery embolization, liver transplant, and antiangiogenic therapy. Bevacizumab has also proven beneficial in improving high output cardiac failure in patients with liver AVMs and has now become first line therapy for liver AVM patients.46 Transplant should be considered in patients with ischemic biliary necrosis, refractory heart failure, or refractory portal hypertension.47 Hepatic artery embolization can be considered in patients who fail bevacizumab and are not candidates for transplant.48
Iron deficiency anemia
Iron deficiency anemia (IDA) is the most common presentation in HHT after epistaxis and telangiectasias.24 Further, iron deficiency in the absence anemia is common and frequently under-diagnosed in HHT. These patients can be symptomatic (fatigue, hair loss, restless leg syndrome, arthralgia, myalgia, poor attention span) and benefit from iron replacement therapy.
Oral iron replacement is frequently ineffective, either because of side effects leading to poor adherence or because iron loss from chronic bleeding exceeds the absorptive capacity of the GI tract. A number of patients therefore require chronic parenteral iron replacement. Blood transfusions may be required in severe cases.
Screening for clinical manifestations and laboratory parameters of IDA should be performed annually in all HHT patients and more frequently in patients with history of IDA. Aggressive replacement of iron (oral or parenteral) should be pursued in parallel with efforts to control bleeding.
Pulmonary hypertension:
Patients with HHT can develop pulmonary hypertension (PH) as a consequence of HHT. PH is not a common complication of HHT and the exact prevalence of PH in HHT has not been established. PH can develop as a primary phenomenon in patents with ACVRL1 mutations or as a consequence of HHT related AVMs.49,50 Patients with symptoms concerning for PH should be managed in collaboration with pulmonology.
Next