Factor X Deficiency
Medications/Treatment
On October 20, 2015, the U.S. Food and Drug Administration (FDA) approved Coagadex®, Coagulation Factor X (Human), for hereditary FX deficiency. Until this orphan drug approval, no specific coagulation factor replacement therapy had been available for patients with hereditary FX deficiency.42-44 Viral inactivation of this plasma derived FX (pdFX) product includes solvent detergent treatment, nanofiltration and terminal heat treatment. The final product contains 100 IU/ml of FX with a specific activity of >100 IU/mg protein. A study of 16 patients with moderate to severe FX deficiency (< 5%) using a dose of 25 IU/kg established a half-life of 29.4 hours. This favorable half-life suggests prophylactic dosing could be administered 1-2 times per week. The observed incremental recovery was 2.00 IU/dl.42-44 In the pivotal Ten01 trial, 16 patients, over a minimum study period of 6-24 months, infused 25 IU/kg of pdFX for 187 bleeds of which approximately half were graded as “major”. Subjective rating for efficacy was “excellent or good” in 98% of subjects. A single infusion was needed in 83% of the bleeds. A small surgical study in 5 patients undergoing seven surgeries reported 100% efficacy.45 No major adverse events have been reported. Inhibitory antibodies were not observed.
In 9 children <12 years treated with Coagadex®, mean incremental recovery at 30 minutes was 1.74 IU/dl per IU/kg, and was significantly lower among subjects aged 0-5 years compared with subjects aged 6-11 years.46
In addition to the newly approved FX concentrate, fresh frozen plasma (FFP) or plasma-derived FIX concentrates (prothrombin complex concentrates [PCC]) may be used for FX replacement therapy. FFP has been associated with allergic reactions and transfusion-associated lung injury. PCC is a plasma-derived concentrate containing three (FII, FIX and FX) or four (FII, FVII, FIX and FX) vitamin K dependent factors with varying amounts of the naturally occurring vitamin K dependent anticoagulants, Proteins C and S. The concentrate undergoes viral inactivation procedures to lessen the risk of viral transmission. The use of PCC in high doses has been associated with thrombosis in hemophilia patients, but the precise frequency is unknown. There have been no reported cases of inhibitory antibodies to FX in patients with congenital FX deficiency in patients treated with FFP, PCC or purified FX.
The biological half-life of infused FX is 20-40 hours, but varies among individuals and with repeated dosing.2 A loading dose of 10-20 mL/kg of fresh frozen plasma, followed by 3-6 mL/kg twice daily, will usually achieve trough levels above 10-20%.30 PCC or highly purified FIX concentrates may contain therapeutic amounts of FX as well (Table 6). PCC products with a FX:FIX ratio of 1:1 will increase plasma levels approximately 1.5% for every 1 IU/kg BW given. The FX concentrate, Factor X P Behring, has an in vivo recovery of 3.3 in patients over 12 years of age and a terminal half-life of 24 hours.47 Because of the long half-life, daily treatment may result in increasing levels and are not usually required.19 Monitoring FX and FIX levels is required during long-term treatment or in the postoperative period to avoid overtreatment and risk of thrombosis.
Adjuvant use of antifibrinolytic therapy should be avoided given the theoretical concern for thrombosis.
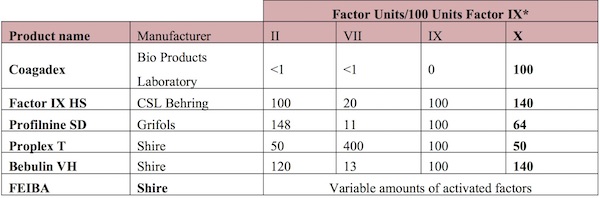
Table 5 summarizes the commercial products containing FX.
* except for Coagadex, the factor unit is not based on FIX but rather contains 100 IU/ml FX
Modified from: Roberts, H.R. and M.D. Bingham, Other Coagulation Factor Deficiencies. Thrombosis and Hemorrhage, 1998. Williams & Wilkins, Baltimore, MD, 2nd Edition: p. 773-802. Source: Brown DL, Kouides PA. Diagnosis and treatment of inherited factor X deficiency. Haemophilia. 2008;14, 1176–1182.
Topical Therapies and Antifibrinolytic Agents
For minor bleeding symptoms, topical and antifibrinolytic agents may be adequate. Topical powders for nosebleeds may be helpful in the treatment of epistaxis, and fibrin glue preparations can be used at surgical sites to achieve local hemostasis. Aminocaproic acid (Amicar®) can be used as a mouthwash (15 mL every 6 hours) or can be taken orally (50-100 mg/kg, maximum of 3g every 6 hours) for oral bleeding or recurrent epistaxis. Aminocaproic acid is also reported to be effective in the treatment of idiopathic menorrhagia and is used with generally good results in women with bleeding disorders. Tranexamic acid is a better tolerated and more potent antifibrinolytic agent,48 (oral dose is 15mg/kg or 1g every 6-8 hours). An oral preparation of tranexamic acid, Lysteda®, at a dose of 1.3g tid has been licensed in the United States and the European Union for use in women with menorrhagia.49
Activated recombinant factor VII (rFVIIa) has been reported to be effective in treating persistent skin and muscle bleeding in a patient with acquired FX deficiency associated with amyloidosis.29 Because FX is a substrate for rFVIIa, it has been suggested that rFVIIa may prove ineffective in cases of severe FX deficiency.20
With early recognition and diagnosis of severe FX deficiency, bleeding symptoms can be effectively treated and managed. Because of the rarity of FX deficiency, evidence-based management guidelines are lacking. The United Kingdom Haemophilia Centre Doctors’ Organisation has published guidelines for management of FX deficiency and other rare coagulation disorders based on literature and extensive clinical experience.20
Targeted levels for treatment and surgery are not well established. Patients with FX levels >10% and no significant bleeding history may not require treatment.19 In a single case report from 1985, FX levels of 9-17% achieved with FFP were sufficient to control minor bleeding.50 Emergency surgery for hemoperitoneum was safely performed with the use of PCC to achieve a FX level of 35%, followed by FFP to maintain FX levels of 10-20% for 6 days in the postoperative period.50 A subdural hematoma was evacuated in an infant after treatment with 10 mL/kg of plasma every 8 hours for 10 days.51 A central venous catheter was placed without bleeding complications using PCC (40-80 IU/kg given on alternate days) which maintained FX levels above 50%.20
Although prophylactic replacement therapy has been used by FVIII and FIX deficient patients to prevent recurrent hemarthrosis and ICH, these strategies have only recently been attempted for FX deficient patients. Certain genotypes appear to be associated with a higher rate of hemarthrosis and ICH, and this information could influence the decision to initiate prophylactic therapy. Kouides and Kulzer reported a patient with recurrent hemarthrosis treated with Profilnine® SD (FX:FIX ratio of 0.5-1.0, 30 FIX IU/kg, twice weekly), who had only a single trauma-induced bleeding episode during the 23-month follow-up period.52 A trough FX level drawn 48 hours after infusion was 30%, and the patient had no thrombotic complications.
Seven patients in the Greifswald Factor X Deficiency Registry have initiated prophylactic therapy for joint disease. They were treated with FIX HS (Behring, 15-20 FX IU/kg) once weekly.53 Dosing was increased to 2-3 times weekly to prevent breakthrough bleeding, and 2 patients required every other day treatment. Among an Iranian cohort, 10 patients received prophylaxis with Factor X P (Behring, 20 FX IU/kg) once weekly with a 1-year follow-up. This regimen was generally well tolerated and resulted in no bleeding symptoms, with only one discontinuation due to anaphylaxis.47
A 21-month-old child with recurrent intracranial hemorrhage was given PCC (40 FIX IU/kg) twice weekly and had a pre-infusion FX level of 7% and a post-infusion FX level of 85% with no further bleeding while following this regimen.51 However, in 2 other young children with ICH, PCC given weekly or twice weekly was insufficient to prevent recurrent ICH.54 Four Irish children with severe FX deficiency and history of bleeding symptoms treated with PCC (Bpl 9A, Prothromplex®, 70FIX IU/kg) 1-2 times per week, had no breakthrough bleeding when FX levels were maintained over 5%.55 Survival studies with 2 of the children found a half-life of 16.7-27 hours. The authors suggested that dosing could be based on pharmacokinetics.
Because of a high risk of intracranial hemorrhage among infants with very low FX levels (<1%) caused by homozygous mutations and history of umbilical or gastrointestinal bleeding, early prophylaxis to prevent intracranial hemorrhage should be considered.56
Nine children <12 years of age with FX levels <5% were treated with pdFX (BPL) concentrate 40-50 IU/kg twice per week and adjusted to maintain FX level >5%. Nine bleeds occurred among 3 patients and only 3 were considered major (2-nosebleed, 1-menorrhagia). All responded to a single dose of pdFX and there were no treatment-related adverse events reported. Investigators rated pdFX efficacy excellent for all subjects.46
Among 24 pregnancies in women with severe FX deficiency reported in the literature, 2 women with 4 pregnancies received prophylaxis with PCC beginning at 6 to 20-weeks gestation to treat bleeding complications.36 Minor bleeding symptoms were encountered, but the pregnancies were successful with no major bleeding complications. Of the 24 pregnancies, 15 were treated with PCCs, FFP, or plasma exchange at the time of delivery, and 2 resulted in postpartum hemorrhage that was not considered life threatening. Two of the three women who did not receive treatment prior to delivery experienced life-threatening postpartum hemorrhage requiring transfusion or hysterectomy. None of the women were reported to have had thrombosis.
Recommendations for management of women with severe FX deficiency through the reproductive years have been presented by Nance et al.36 Treatment begins at menarche with hormonal contraceptives or tranexamic acid to reduce menstrual blood flow. Treatment with pdFX has a reported 98% treatment success rate in the treatment of heavy menstrual bleeds in women and girls >12 years with FX levels <5%.57 Prophylactic infusions of pdFX,58 PCC, or FFP during pregnancy should be considered if there are systemic bleeding symptoms, vaginal bleeding, retroplacental hematoma, or history of fetal loss. A scheduled or caesarean delivery facilitates coordination of obstetrics, hematology, and the hospital pharmacy and should be considered on an individual basis. Factor replacement with pdFX (25 IU/kg), FFP (10-15 ml/kg), or PCC (20-40 IU/kg) should be administered at the onset of labor. Circulating FX levels measured 30 minutes post-infusion of 20-40 FX IU/dl are targeted.