Factor V Deficiency
Medications/Treatment
Overall, the prognosis for most FV-deficient patients is good. None of the patients in the North American Registry, including those with FV activity <1%, required prophylaxis;24 and the Iranian cohort appeared to have a more benign course than patients with hemophilia A and B with comparable deficiency levels.27 The most severe cases appear to be patients who present in the perinatal period with intracranial hemorrhage.23,31,33 Owren’s index case died in 2002 at the age of 88 years.3
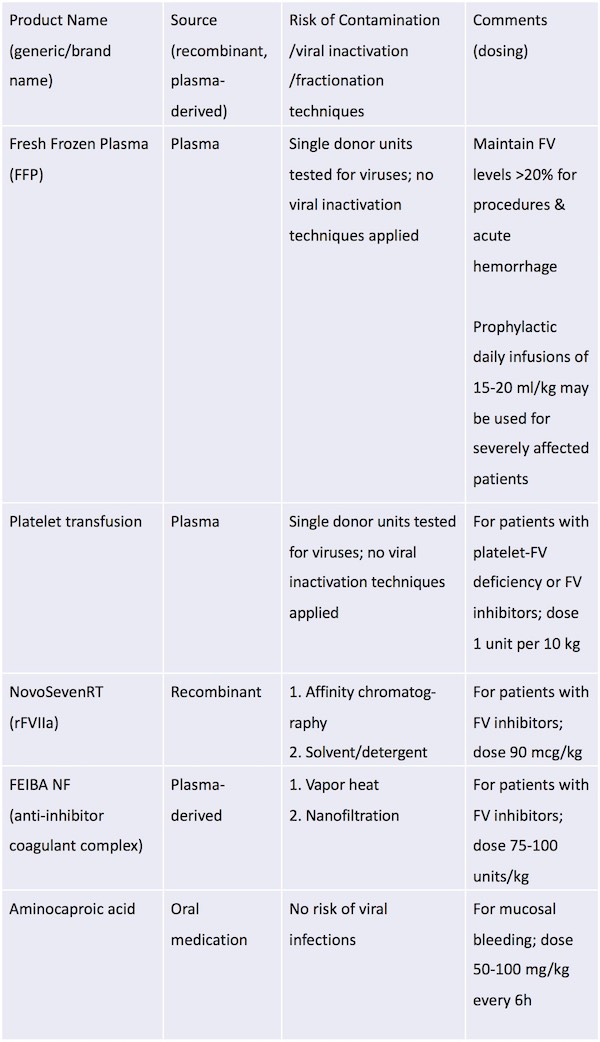
Fresh frozen plasma (FFP) is the primary therapeutic option because no FV-specific concentrate is available. For less severe mucosal bleeding, antifibrinolytic agents such as aminocaproic acid may be sufficient.24
Most patients are treated episodically for bleeding and before invasive procedures.24 However, case reports are available of severely affected patients presenting early in life who require routine prophylactic FFP infusions.31,33,39 For procedures and for acute hemorrhage, the goal of therapy is to maintain FV levels above 20%. The half-life of FV is 12–36h, and, typically, daily infusions of 15–20 mL kg–1 of FFP are sufficient.27,28 However, the frequency and dosing should be adjusted based on FV levels required to achieve hemostasis. Aside from concerns for potential allergic reactions and infection, treatment with FFP has the additional risk of volume overload. Plasma exchange has been successfully used to circumvent this complication.40
A possible alternative to FFP is recombinant FVIIa (rFVIIa; NovoSeven® RT),41,42 which is currently approved for use in patients with FVII deficiency and in patients who have hemophilia A and B with inhibitors. However, this product has been used off-label to treat bleeding as a result of liver disease and warfarin-associated bleeding or overdose. The mechanism of action in these settings is unknown but is thought to be related to the large dose of activated FVII. Thus, it is theoretically possible that this product may treat bleeding in patients with FV deficiency, and it has been reported to be effective in patients with severe FV deficiency.41,43 The advantages of rFVIIa are the lower volume of infusion and, as a recombinant product, the lack of risk of human viral infections. However, the disadvantages are the unknown mechanism of action and the unknown dose; excessive dosing may place the patient at risk for excessive clotting.
The activated prothrombin complex concentrate FEIBA® (anti-inhibitor coagulant complex), another FVIII-inhibitor bypassing agent, is less likely to be of benefit because it works by virtue of the varying amount of activated Factor X infused; it also has a greater risk of thrombosis than rFVIIa because of the presence of multiple species of activated clotting factors. The volume of FEIBA is less than FFP but greater than rFVIIa; the viral risk is also less than with FFP but greater than with rFVIIa, as FEIBA is a plasma-derived product.
A Factor V concentrate has been developed commercially but is not currently FDA-approved.
In patients with platelet-FV deficiency and those with FV inhibitors, transfusion with platelets rather than FFP and/or bypassing agents may be beneficial as platelets contain adequate amounts of FVa.44,45 The dose is 1 unit of platelets per 10 kg body weight; half-life of the platelets is 3-5 days, and the risks are alloimmunization, allergic reaction, and infection.
Management of Menorrhagia and Pregnancy
Women with severe manifestations of FV deficiency require special consideration for issues of menstruation and pregnancy. Hormonal therapy may be useful for management of menorrhagia. FV-deficient patients should receive FFP at the time of delivery, and pregnancies managed with this approach have been shown to have good outcomes.46 Successful management using FFP infusions has been described for a woman undergoing fertility procedures47 and for a woman with hemorrhagic ovarian cysts leading to hemoperitoneum.42 A recent report from Iran details the successful management of pregnancy, labor and delivery with prophylactic infusions of FFP throughout the pregnancies and deliveries in five severely affected women (FV <1%) with history of recurrent fetal loss.48Treatment of FV Inhibitors
Rarely, FV-deficient patients have developed inhibitors to FV after receiving FFP.6,23,24 For such patients, activated prothrombin complex concentrate (FEIBA) and rFVIIa concentrate are options. Platelet transfusions may provide a source of FV that is more resistant to inhibition by the circulating antibodies.49